
Studies have shown that the Cr(III) supplementation in deficient and marginally deficient subjects can result in the rapid reversal of many of the symptoms of chromium-deficiency. The atomic number of chromium is 24, so we know that a neutral chromium atom has 24 protons and 24 electrons. This acid is composed of two ions, hydrogen ions, and polyatomic chromate. The biologically active form of an organic Cr(III) complex, often referred to as glucose tolerance factor (GTF), is believed to function by facilitating the interaction of insulin with its cellular receptor sites. The chromic acid formula is H 2 C r O 4 its molecule is composed of two hydrogens, four oxygens, and a chromium atom. Therefore, many people’s diets may not provide enough Cr(III).
#CHROMIUM ATOM FULL#
However, the reason for this specific configuration is that chromium is more stable with half filled 4s and 3d orbitals than only a 40 full 3d orbital.

Chromium Basic Facts Chromium Atomic Number: 24 Chromium Symbol: Cr Chromium Atomic Weight: 51.

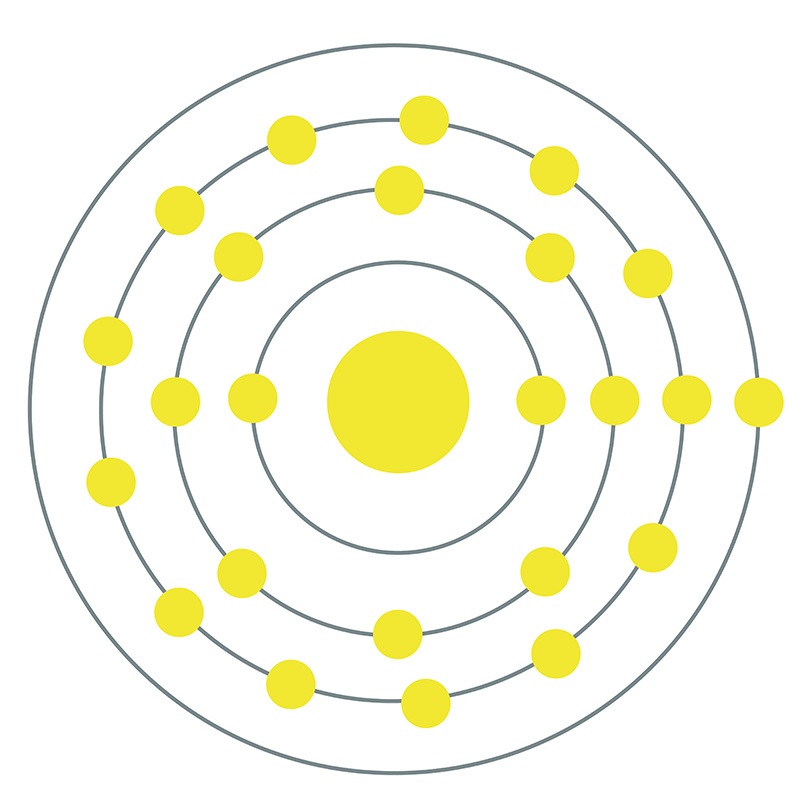
The electron configuration of a chromium atom is 1s2 2s2 2p6 3s2 3p6 4s1 3d5. Chromium is element atomic number 24 with element symbol Cr. Edge Length Of The Unit Cell is Found to Be 287 Pm. On the average, adults in the United States take in an estimated 60-80 micrograms of Cr(III) per day in food. Chromium is the 24th element in the periodic table and its symbol is ‘Cr’. Explanation: This is an anomalous configuration, something silly that you shouldnt be tested on, in my opinion. A chromium atom has four electron shells. The National Academy of Sciences has established a safe and adequate daily intake for Cr(III) in adults of 50-200 micrograms per day. Name of the simple mixer control element that the ALSA-based media library should use to mute the system. Other significant sources of Cr(III) are mineral supplements, brewer’s yeast, and beer. If this atom loses one electron, it will become a cation with a 1+ charge (11 10 1+). For example, a neutral sodium atom (Z 11) has 11 electrons. Positively charged atoms called cations are formed when an atom loses one or more electrons. Cr(III) deficiency has been associated withĬr(III) is found in most fresh foods and drinking water. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. It is required to potentiate insulin and for normal glucose metabolism. Cr(III) is an essential dietary nutrient.


 0 kommentar(er)
0 kommentar(er)
